History of atomic theory
[4] Working in the late 17th century, Robert Boyle developed the concept of a chemical element as substance different from a compound.
This brought an end to the ancient idea of the elements of matter being fire, earth, air, and water, which had no experimental support.
Aside from the crudity of early 19th century measurement tools, the main reason for this error was that Dalton didn't know that the water molecule in fact has two hydrogen atoms, not one.
This way of thinking led directly to a second hypothesis: the particles of certain elemental gases were pairs of atoms, and when reacting chemically these molecules often split in two.
The various quantities of a particular element involved in the constitution of different molecules are integral multiples of a fundamental quantity that always manifests itself as an indivisible entity and which must properly be named atom.Cannizzaro criticized past chemists such as Berzelius for not accepting that the particles of certain gaseous elements are actually pairs of atoms, which led to mistakes in their formulation of certain compounds.
[2]: 237 These positions were eventually quashed by two important advancements that happened later in the 19th century: the development of the periodic table and the discovery that molecules have an internal architecture that determines their properties.
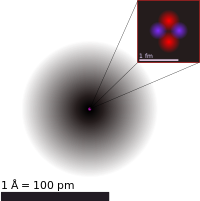
The modern periodic table is based on atomic number, which is equivalent to the nuclear charge, a change had to wait for the discovery of the nucleus.
[37]: 222 In 1738, Swiss physicist and mathematician Daniel Bernoulli postulated that the pressure of gases and heat were both caused by the underlying motion of particles.
"[citation needed] Boltzmann defended the atomistic hypothesis against major detractors from the time like Ernst Mach or energeticists like Wilhelm Ostwald, who considered that energy was the elementary quantity of reality.
[40] At the beginning of the 20th century, Albert Einstein independently reinvented Gibbs' laws, because they had only been printed in an obscure American journal.
[citation needed] In 1827, the British botanist Robert Brown observed that dust particles inside pollen grains floating in water constantly jiggled about for no apparent reason.
In 1905, Einstein theorized that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a mathematical model to describe it.
This model was validated experimentally in 1908 by French physicist Jean Perrin, who used Einstein's equations to measure the size of atoms.
When a voltage is applied across the electrodes, cathode rays are generated, creating a glowing patch where they strike the glass at the opposite end of the tube.
[45] In contrast to those corpuscles, positive ions created by electrolysis or X-ray radiation had mass-to-charge ratios that varied depending on the material of the electrodes and the type of gas in the reaction chamber, indicating they were different kinds of particles.
[37]: 85 [46] In 1899, he showed that negative electricity created by ultraviolet light landing on a metal (known now as the photoelectric effect) has the same mass-to-charge ratio as cathode rays; then he applied his previous method for determining the charge on ions to the negative electric particles created by ultraviolet light.
[52] The analogy suggests that the positive sphere is like a solid, but Thomson likened it to a liquid, as he proposed that the electrons moved around in it in patterns governed by the electrostatic forces.
Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments.
[55] Between 1908 and 1913, Rutherford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with a beam of alpha particles.
Only such an intense concentration of charge, anchored by its high mass, could produce an electric field strong enough to deflect the alpha particles as observed.
Geiger and Marsden's based their analysis on setting the charge to half of the atomic weight of the foil's material (gold, aluminium, etc.).
Amateur physicist Antonius van den Broek noted that there was a more precise relation between the charge and the element's numeric sequence in the order of atomic weights.
[37]: 19 Max Planck in 1900 and Albert Einstein in 1905 had postulated that light energy is emitted or absorbed in discrete amounts known as quanta (singular, quantum).
[62] Worse still, it could not even account for all features of the hydrogen spectrum: as spectrographic technology improved, it was discovered that applying a magnetic field caused spectral lines to multiply in a way that Bohr's model couldn't explain.
[66] That same year, J. J. Thomson conducted an experiment in which he channeled a stream of neon ions through magnetic and electric fields, striking a photographic plate at the other end.
Rutherford concluded that the alpha particles struck the nuclei of the nitrogen atoms, causing hydrogen ions to split off.
Before the discovery of the neutron, scientists believed that the atomic nucleus contained a number of "nuclear electrons" which cancelled out the positive charge of some of its protons.
Rutherford wrote that the existence of such "neutral doublets" moving freely through space would provide a more plausible explanation for how the heavier elements could have formed in the genesis of the Universe, given that it is hard for a lone proton to fuse with a large atomic nucleus because of the repulsive electric field.
[80] In 1928, Walter Bothe observed that beryllium emitted a highly penetrating, electrically neutral radiation when bombarded with alpha particles.
This approach predicted many of the spectral phenomena that Bohr's model failed to explain, but it was difficult to visualize, and faced opposition.

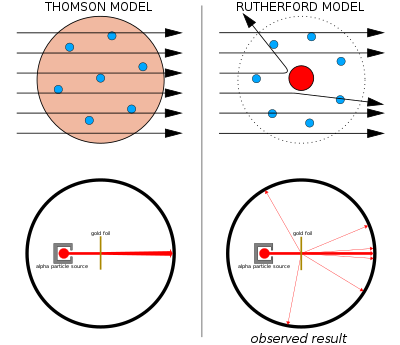
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.

