Iron
Iron also forms many coordination complexes; some of them, such as ferrocene, ferrioxalate, and Prussian blue have substantial industrial, medical, or research applications.
Iron is also the metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals.
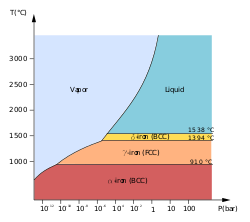
[11] The physical properties of iron at very high pressures and temperatures have also been studied extensively,[12][13] because of their relevance to theories about the cores of the Earth and other planets.
Some controversial experimental evidence exists for a stable β phase at pressures above 50 GPa and temperatures of at least 1500 K. It is supposed to have an orthorhombic or a double hcp structure.
Impurities, lattice defects, or grain and particle boundaries can "pin" the domains in the new positions, so that the effect persists even after the external field is removed – thus turning the iron object into a (permanent) magnet.
In the last decade, advances in mass spectrometry have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the stable isotopes of iron.
[24] In phases of the meteorites Semarkona and Chervony Kut, a correlation between the concentration of 60Ni, the granddaughter of 60Fe, and the abundance of the stable iron isotopes provided evidence for the existence of 60Fe at the time of formation of the Solar System.
Element production in supernovas greatly favor iron over nickel, and in any case, 56Fe still has a lower mass per nucleon than 62Ni due to its higher fraction of lighter protons.
[49][50] Materials containing finely ground iron(III) oxides or oxide-hydroxides, such as ochre, have been used as yellow, red, and brown pigments since pre-historical times.
[51] Through Eisensandstein (a jurassic 'iron sandstone', e.g. from Donzdorf in Germany)[52] and Bath stone in the UK, iron compounds are responsible for the yellowish color of many historical buildings and sculptures.
[59] Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity.
[68] Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:[68] Ferric iodide, a black solid, is not stable in ordinary conditions, but can be prepared through the reaction of iron pentacarbonyl with iodine and carbon monoxide in the presence of hexane and light at the temperature of −20 °C, with oxygen and water excluded.
For example, the trans-chlorohydridobis(bis-1,2-(diphenylphosphino)ethane)iron(II) complex is used as a starting material for compounds with the Fe(dppe)2 moiety.
[78] A landmark in this field was the discovery in 1951 of the remarkably stable sandwich compound ferrocene Fe(C5H5)2, by Pauson and Kealy[79] and independently by Miller and colleagues,[80] whose surprising molecular structure was determined only a year later by Woodward and Wilkinson[81] and Fischer.
[86] The technology developed slowly, and even after the discovery of smelting it took many centuries for iron to replace bronze as the metal of choice for tools and weapons.
[110] Medieval blast furnaces were about 10 feet (3.0 m) tall and made of fireproof brick; forced air was usually provided by hand-operated bellows.
[108] Modern blast furnaces have grown much bigger, with hearths fourteen meters in diameter that allow them to produce thousands of tons of iron each day, but essentially operate in much the same way as they did during medieval times.
In the late 1850s, Henry Bessemer invented a new steelmaking process, involving blowing air through molten pig iron, to produce mild steel.
[115] The Iron Age was closely related with Rome, and in Ovid's Metamorphoses The Virtues, in despair, quit the earth; and the depravity of man becomes universal and complete.
In the first stage, iron ore is reduced with coke in a blast furnace, and the molten metal is separated from gross impurities such as silicate minerals.
The blast furnace is loaded with iron ores, usually hematite Fe2O3 or magnetite Fe3O4, along with coke (coal that has been separately baked to remove volatile components) and flux (limestone or dolomite).
[120] The pig iron produced by the blast furnace process contains up to 4–5% carbon (by mass), with small amounts of other impurities like sulfur, magnesium, phosphorus, and manganese.
Its low cost and high strength often make it the material of choice to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings.
Cooling a mixture of iron with 0.8% carbon slowly below 723 °C to room temperature results in separate, alternating layers of cementite and α-iron, which is soft and malleable and is called pearlite for its appearance.
Iron(III) chloride finds use in water purification and sewage treatment, in the dyeing of cloth, as a coloring agent in paints, as an additive in animal feed, and as an etchant for copper in the manufacture of printed circuit boards.
[10][157] A major component of this regulation is the protein transferrin, which binds iron ions absorbed from the duodenum and carries it in the blood to cells.
[150] When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe2+ ion at the center of the heme group (in the hydrophobic protein interior) is in a high-spin configuration.
The sixth coordination site is then occupied by either another imidazole nitrogen or a methionine sulfur, so that these proteins are largely inert to oxygen—with the exception of cytochrome a, which bonds directly to oxygen and thus is very easily poisoned by cyanide.
[167] The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for iron in 2001.
Most cases of iron-deficiency anemia are mild, but if not treated can cause problems like fast or irregular heartbeat, complications during pregnancy, and delayed growth in infants and children.













carbonyl