Nucleotide
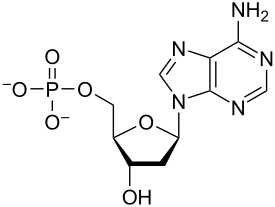
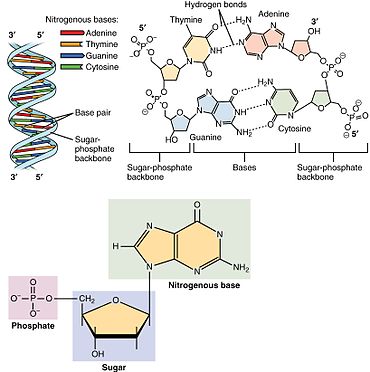
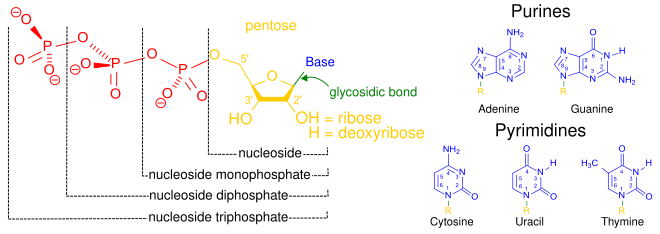
[3] A nucleotide is composed of three distinctive chemical sub-units: a five-carbon sugar molecule, a nucleobase (the two of which together are called a nucleoside), and one phosphate group.
In a double helix, the two strands are oriented in opposite directions, which permits base pairing and complementarity between the base-pairs, all which is essential for replicating or transcribing the encoded information found in DNA.
In addition to being building blocks for the construction of nucleic acid polymers, singular nucleotides play roles in cellular energy storage and provision, cellular signaling, as a source of phosphate groups used to modulate the activity of proteins and other signaling molecules, and as enzymatic cofactors, often carrying out redox reactions.
A purified nucleoside is protected to create a phosphoramidite, which can then be used to obtain analogues not found in nature and/or to synthesize an oligonucleotide.
[1] The components used in de novo nucleotide synthesis are derived from biosynthetic precursors of carbohydrate and amino acid metabolism, and from ammonia and carbon dioxide.
Pyrimidines are synthesized first from aspartate and carbamoyl-phosphate in the cytoplasm to the common precursor ring structure orotic acid, onto which a phosphorylated ribosyl unit is covalently linked.
For reference, the syntheses of the purine and pyrimidine nucleotides are carried out by several enzymes in the cytoplasm of the cell, not within a specific organelle.
[citation needed] The synthesis of the pyrimidines CTP and UTP occurs in the cytoplasm and starts with the formation of carbamoyl phosphate from glutamine and CO2.
The reaction is unusual in that a pyrophosphoryl group is directly transferred from ATP to C1 of R5P and that the product has the α configuration about C1.
In the first reaction unique to purine nucleotide biosynthesis, PPAT catalyzes the displacement of PRPP's pyrophosphate group (PPi) by an amide nitrogen donated from either glutamine (N), glycine (N&C), aspartate (N), folic acid (C1), or CO2.
Next, a glycine is incorporated fueled by ATP hydrolysis, and the carboxyl group forms an amine bond to the NH2 previously introduced.
A one-carbon unit from folic acid coenzyme N10-formyl-THF is then added to the amino group of the substituted glycine followed by the closure of the imidazole ring.
Finally, a second one-carbon unit from formyl-THF is added to the nitrogen group and the ring is covalently closed to form the common purine precursor inosine monophosphate (IMP).
RNA is composed of purine and pyrimidine nucleotides, both of which are necessary for reliable information transfer, and thus Darwinian evolution.
Becker et al. showed how pyrimidine nucleosides can be synthesized from small molecules and ribose, driven solely by wet-dry cycles.
5'-mono- and di-phosphates also form selectively from phosphate-containing minerals, allowing concurrent formation of polyribonucleotides with both the purine and pyrimidine bases.
Thus a reaction network towards the purine and pyrimidine RNA building blocks can be established starting from simple atmospheric or volcanic molecules.
[10] An unnatural base pair (UBP) is a designed subunit (or nucleobase) of DNA which is created in a laboratory and does not occur in nature.
These artificial nucleotides bearing hydrophobic nucleobases, feature two fused aromatic rings that form a (d5SICS–dNaM) complex or base pair in DNA.
[12][15] The applications of synthetic nucleotides vary widely and include disease diagnosis, treatment, or precision medicine.
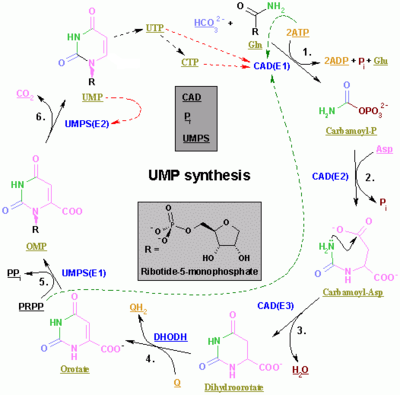
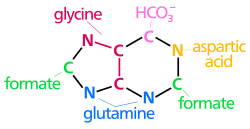
N 1 arises from the amine group of Asp
C 2 and C 8 originate from formate
N 3 and N 9 are contributed by the amide group of Gln
C 4 , C 5 and N 7 are derived from Gly
C 6 comes from HCO 3 − (CO 2 )
