History of chemistry
[14]In multiple Warring States period tombs, sharp swords and other weapons were also found to be coated with 10 to 15 micrometers of chromium oxide, which left them in pristine condition to this day.
In fact, according to The Fontana History of Chemistry (Brock, 1992): The language of alchemy soon developed an arcane and secretive technical vocabulary designed to conceal information from the uninitiated.
To a large degree, this language is incomprehensible to us today, though it is apparent that readers of Geoffery Chaucer's Canon's Yeoman's Tale or audiences of Ben Jonson's The Alchemist were able to construe it sufficiently to laugh at it.
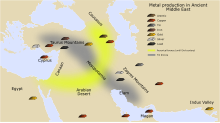
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them Georg Agricola (1494–1555), who published his great work De re metallica in 1556.
Working during the time just after Paracelsus and iatrochemistry, Jan Baptist van Helmont suggested that there are insubstantial substances other than air and coined a name for them – "gas", from the Greek word chaos.
Jan Baptist van Helmont is also remembered today largely for his ideas on spontaneous generation and his 5-year tree experiment, as well as being considered the founder of pneumatic chemistry.
A Swedish chemist and disciple of Dalton, Jöns Jacob Berzelius embarked on a systematic program to try to make accurate and precise quantitative measurements and to ensure the purity of chemicals.
English chemist Humphry Davy was a pioneer in the field of electrolysis, using Alessandro Volta's voltaic pile to split up common compounds and thus isolate a series of new elements.
Chlorine was discovered in 1774 by Swedish chemist Carl Wilhelm Scheele, who called it "dephlogisticated marine acid" (see phlogiston theory) and mistakenly thought it contained oxygen.
Although the idea of the safety lamp had already been demonstrated by William Reid Clanny and by the then unknown (but later very famous) engineer George Stephenson, Davy's use of wire gauze to prevent the spread of flame was used by many other inventors in their later designs.
During the 1850s, younger chemists, such as Alexander Williamson in England, Charles Gerhardt and Charles-Adolphe Wurtz in France, and August Kekulé in Germany, began to advocate reforming theoretical chemistry to make it consistent with Avogadrian theory.
[88] Markovnikov's rule was an early example of regioselectivity in organic synthesis and the modern understanding of it continues to be important in the chemical industry, where catalysts have been developed to produce anti-Markovnikov products.
In 1856, Sir William Henry Perkin, age 18, given a challenge by his professor, August Wilhelm von Hofmann, sought to synthesize quinine, the anti-malaria drug, from coal tar.
Swedish chemist and inventor Alfred Nobel found that when nitroglycerin was incorporated in an absorbent inert substance like kieselguhr (diatomaceous earth) it became safer and more convenient to handle, and this mixture he patented in 1867 as dynamite.
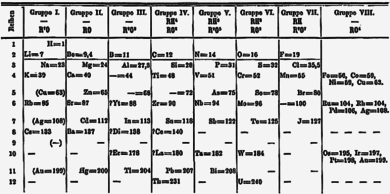
Mendeleev, a Russian chemist, felt that there was some type of order to the elements and he spent more than thirteen years of his life collecting data and assembling the concept, initially with the idea of resolving some of the disorder in the field for his students.
German engineer Carl von Linde's invention of a continuous process of liquefying gases in large quantities formed a basis for the modern technology of refrigeration and provided both impetus and means for conducting scientific research at low temperatures and very high vacuums.
His pressure laws, given general validity by the electrolytic dissociation theory of Arrhenius (1884–1887) – the first foreigner who came to work with him in Amsterdam (1888) – are considered the most comprehensive and important in the realm of natural sciences.
Using two different methods to remove all known gases from air, Ramsay and Lord Rayleigh were able to announce in 1894 that they had found a monatomic, chemically inert gaseous element that constituted nearly 1 percent of the atmosphere; they named it argon.
Sir William Ramsay worked with Frederick Soddy to demonstrate, in 1903, that alpha particles (helium nuclei) were continually produced during the radioactive decay of a sample of radium.
While working with Marie to extract pure substances from ores, an undertaking that really required industrial resources but that they achieved in relatively primitive conditions, Pierre himself concentrated on the physical study (including luminous and chemical effects) of the new radiations.
Beginning in 1912, he spent several years investigating and finally proving Albert Einstein's proposed linear relationship between energy and frequency, and providing the first direct photoelectric support for the Planck constant.
The peak of Frederick Soddy's career in radiochemistry was in 1913 with his formulation of the concept of isotopes, which stated that certain elements exist in two or more forms which have different atomic weights but which are indistinguishable chemically.
His renewal of interest in this subject was largely stimulated by the activities of the American chemist and General Electric researcher Irving Langmuir, who between 1919 and 1921 popularized and elaborated Lewis's model.
However, though some principles deduced from quantum mechanics were able to predict qualitatively some chemical features for biologically relevant molecules, they were, till the end of the 20th century, more a collection of rules, observations, and recipes than rigorous ab initio quantitative methods.
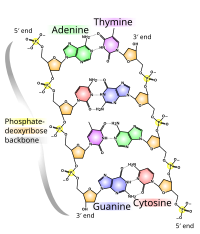
[citation needed] This heuristic approach triumphed in 1953 when James Watson and Francis Crick deduced the double helical structure of DNA by constructing models constrained by and informed by the knowledge of the chemistry of the constituent parts and the X-ray diffraction patterns obtained by Rosalind Franklin.
This first attempt by chemists to study hypothetical processes in the laboratory under controlled conditions helped kickstart bountiful research, within the natural sciences, into the origins of life.
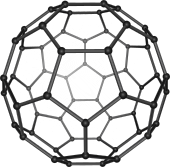
[118][119][120] In 1985, Harold Kroto, Robert Curl and Richard Smalley discovered fullerenes, a class of large carbon molecules superficially resembling the geodesic dome designed by architect R. Buckminster Fuller.
Though they developed a consistent quantitative foundation based on notions of atomic and molecular weights, combining proportions, and thermodynamic quantities, chemists had less use of advanced mathematics.
(See Philosophy of chemistry) The later part of the nineteenth century saw a huge increase in the exploitation of petroleum extracted from the earth for the production of a host of chemicals and largely replaced the use of whale oil, coal tar and naval stores used previously.
[citation needed] In the mid-twentieth century, control of the electronic structure of semiconductor materials was made precise by the creation of large ingots of extremely pure single crystals of silicon and germanium.