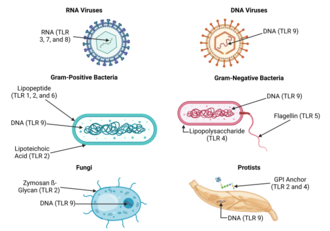
Toll-like receptor
Toll-like receptors (TLRs) are a class of proteins that play a key role in the innate immune system.
Once these microbes have reached physical barriers such as the skin or intestinal tract mucosa, they are recognized by TLRs, which activate immune cell responses.
[5] The binding of ligands — either in the form of adjuvant used in vaccinations or in the form of invasive moieties during times of natural infection — to the TLR marks the key molecular events that ultimately lead to innate immune responses and the development of antigen-specific acquired immunity.
Toll-like receptors have also been shown to be an important link between innate and adaptive immunity through their presence in dendritic cells.
In addition to the recognition of exogenous PAMPs, TLRs can also bind to endogenous damage-associated molecular patterns (DAMPs) such as heat shock proteins (HSPs) or plasma membrane constituents.
Proteins with subgroup 2 TIR domains are classical TLRs, and bind directly or indirectly to molecules of microbial origin.
Members of the TLR family were detected on glia, neurons and on neural progenitor cells in which they regulate cell-fate decision.
Thirteen TLRs (named simply TLR1 to TLR13) have been identified in humans and mice together, and equivalent forms of many of these have been found in other mammalian species.
For example, a gene coding for a protein analogous to TLR10 in humans is present in mice, but appears to have been damaged at some point in the past by a retrovirus.
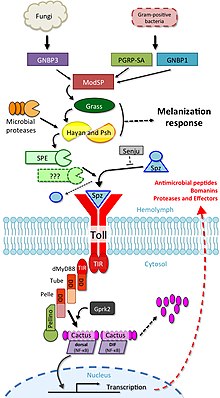
Its receptor ectodomain recognizes the cleaved form of the cytokine spätzle, which is secreted in the haemolymph as an inactive dimeric precursor.
The toll receptor shares the cytoplasmatic TIR domain with mammalian TLRs, but the ectodomain and intracytoplasmatic tail are different.
Signal from TICS is then transduced to Cactus (homologue of mammalian IκB), phosphorylated Cactus is polyubiquitylated and degraded, allowing nuclear translocation of DIF (dorsal-related immunity factor; a homologue of mammalian NF-κB) and induction of transcription of genes for antimicrobial peptides (AMPs) such as drosomycin.
Pathogen-associated molecules that meet this requirement are thought to be critical to the pathogen's function and difficult to change through mutation; they are said to be evolutionarily conserved.
TLRs have been suspected of binding to host molecules including fibrinogen (involved in blood clotting), heat shock proteins (HSPs), HMGB1, extracellular matrix components and self DNA (it is normally degraded by nucleases, but under inflammatory and autoimmune conditions it can form a complex with endogenous proteins, become resistant to these nucleases and gain access to endosomal TLRs as TLR7 or TLR9).
Though most TLRs appear to function as homodimers, TLR2 forms heterodimers with TLR1 or TLR6, each dimer having a different ligand specificity.
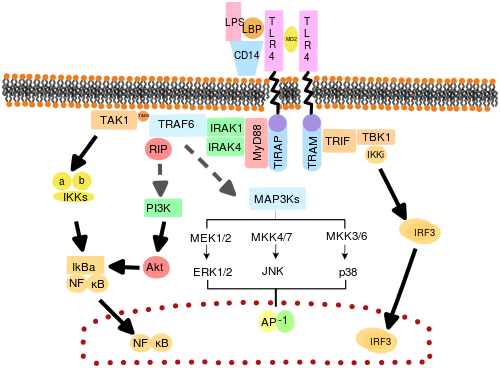
TLRs may also depend on other co-receptors for full ligand sensitivity, such as in the case of TLR4's recognition of LPS, which requires MD-2.
Ligand binding and conformational change that occurs in the receptor recruits the adaptor protein MyD88, a member of the TIR family.
Complex consisting of TLR4, MD2 and LPS recruits TIR domain-containing adaptors TIRAP and MyD88 and thus initiates activation of NFκB (early phase) and MAPK.
TLR7 messenger RNA expression levels in dairy animals in a natural outbreak of foot-and-mouth disease have been reported.
Its activation leads to downstream release of inflammatory modulators including TNF-α and IL-1β, and constant low-level release of these modulators is thought to reduce the efficacy of opioid drug treatment with time, and is involved in opioid tolerance,[58][59] hyperalgesia and allodynia.
A large body of literature, spanning most of the last century, attests to the search for the key molecules and their receptors.
More than 100 years ago, Richard Pfeiffer, a student of Robert Koch, coined the term "endotoxin" to describe a substance produced by Gram-negative bacteria that could provoke fever and shock in experimental animals.
Other molecules (bacterial lipopeptides, flagellin, and unmethylated DNA) were shown in turn to provoke host responses that are normally protective.
It followed logically that there must be receptors for such molecules, capable of alerting the host to the presence of infection, but these remained elusive for many years.
Toll-like receptors are now counted among the key molecules that alert the immune system to the presence of microbial infections.
The prototypic member of the family, the toll receptor (P08953; Tl) in the fruit fly Drosophila melanogaster, was discovered in 1985 by 1995 Nobel Laureates Christiane Nüsslein-Volhard and Eric Wieschaus and colleagues.
[69] In 1996, toll was found by Jules A. Hoffmann and his colleagues to have an essential role in the fly's immunity to fungal infection, which it achieved by activating the synthesis of antimicrobial peptides.
[72] In 1997, Charles Janeway and Ruslan Medzhitov showed that a toll-like receptor now known as TLR4 could, when artificially ligated using antibodies, induce the activation of certain genes necessary for initiating an adaptive immune response.
Each TLR is now believed to detect a discrete collection of molecules — some of microbial origin, and some products of cell damage — and to signal the presence of infections.