Major histocompatibility complex
The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system.
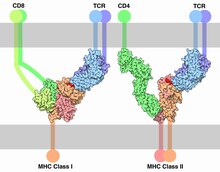
Each MHC molecule on the cell surface displays a small peptide (a molecular fraction of a protein) called an epitope.
Some years later Baruj Benacerraf showed that polymorphic MHC genes not only determine an individual's unique constitution of antigens but also regulate the interaction among the various cells of the immunological system.
These three scientists have been awarded the 1980 Nobel Prize in Physiology or Medicine[10] for their discoveries concerning “genetically determined structures on the cell surface that regulate immunological reactions”.
The IPD-MHC Database[14] was created which provides a centralised repository for sequences of the Major Histocompatibility Complex (MHC) from a number of different species.
The MHC locus is present in all jawed vertebrates; it is assumed to have arisen about 450 million years ago.
In humans, the MHC region occurs on chromosome 6, between the flanking genetic markers MOG and COL11A2 (from 6p22.1 to 6p21.3 about 29Mb to 33Mb on the hg38 assembly), and contains 224 genes spanning 3.6 megabase pairs (3 600 000 bases).
However, historically, the MHC in mice is called the Histocompatibility system 2 or just the H-2, whereas it has been referred to as the RT1 complex in rats, and the B locus in chickens.
The polarization during primary exposure to an antigen is key in determining a number of chronic diseases, such as inflammatory bowel diseases and asthma, by skewing the immune response that memory Th cells coordinate when their memory recall is triggered upon secondary exposure to similar antigens.
MHC is the tissue-antigen that allows the immune system (more specifically T cells) to bind to, recognize, and tolerate itself (autorecognition).
MHC is also the chaperone for intracellular peptides that are complexed with MHCs and presented to T cell receptors (TCRs) as potential foreign antigens.
It is unclear how exactly having the HLA-B27 tissue type increases the risk of ankylosing spondylitis and other associated inflammatory diseases, but mechanisms involving aberrant antigen presentation or T cell activation have been hypothesized.
T cells become activated by binding to the peptide-binding grooves of any MHC molecule that they were not trained to recognize during positive selection in the thymus.
Peptides are processed and presented by two classical pathways: In their development in the thymus, T lymphocytes are selected to recognize the host's own MHC molecules, but not other self antigens.
Positive selection ensures that mature T cells can functionally recognize MHC molecules in the periphery (i.e. elsewhere in the body).
In 1976, Yamazaki et al demonstrated a sexual selection mate choice by male mice for females of a different MHC.
[29] Some data find lower rates of early pregnancy loss in human couples of dissimilar MHC genes.
[33] If it exists, the phenomenon might be mediated by olfaction, as MHC phenotype appears strongly involved in the strength and pleasantness of perceived odour of compounds from sweat.
[34] In 1995, Claus Wedekind found that in a group of female college students who smelled T-shirts worn by male students for two nights (without deodorant, cologne, or scented soaps), the majority of women chose shirts worn by men of dissimilar MHCs, a preference reversed if the women were on oral contraceptives.
For example, relatively low MHC diversity has been observed in the cheetah (Acinonyx jubatus),[47] Eurasian beaver (Castor fiber),[48] and giant panda (Ailuropoda melanoleuca).
[49] In 2007 low MHC diversity was attributed a role in disease susceptibility in the Tasmanian devil (Sarcophilus harrisii), native to the isolated island of Tasmania, such that an antigen of a transmissible tumor, involved in devil facial tumour disease, appears to be recognized as a self antigen.
[50] To offset inbreeding, efforts to sustain genetic diversity in populations of endangered species and of captive animals have been suggested.
In ray-finned fish like rainbow trout, allelic polymorphism in MHC class II is reminiscent of that in mammals and predominantly maps to the peptide binding groove.
[51][52][20] It has been speculated that this type of MHC class I allelic variation contributes to allograft rejection, which may be especially important in fish to avoid grafting of cancer cells through their mucosal skin.
"[54] Genes in this locus are apparently linked to intracellular intrinsic immunity in the basal Metazoan Trichoplax adhaerens.
Any two individuals who are not identical twins, triplets, or higher order multiple births, will express differing MHC molecules.
All MHC molecules can mediate transplant rejection, but HLA-C and HLA-DP, showing low polymorphism, seem least important.
[clarification needed] When maturing in the thymus, T lymphocytes are selected for their TCR incapacity to recognize self antigens, yet T lymphocytes can react against the donor MHC's peptide-binding groove, the variable region of MHC holding the presented antigen's epitope for recognition by TCR, the matching paratope.
The polymorphism is so high, in a mixed population (nonendogamic), no two individuals have exactly the same set of MHC molecules, with the exception of identical twins.
Because of the high levels of allelic diversity found within its genes, MHC has also attracted the attention of many evolutionary biologists.